2026
Locquet, P.; Boussalem, N.; Hatey, D.; Grellepois, F.; Hénon, E.; Riguet, E.; Blanc, A. 2026, submitted.
Pre-Print: 10.26434/chemrxiv-2025-2p3qw
2025
Pascaretti, M; Starck, E.; Padilla Hernandez, A.; Pertschi, R, Taillier, C.; Blanc, A.; Weibel, J.-M.; Dalla, V.; Pale, P. Chem. Eur. J. 2025, e202502027.
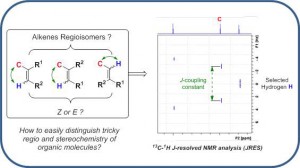
Pereira, P.; Locquet, P.; Blanc, A.; Grellepois, F.; Riguet, E. J. Org. Chem. 2025, 3475–3479.
Pre-Print: 10.26434/chemrxiv-2024-nrz6q
Heinrich, R.; Marie-Rose, G.; Gourlaouen, C.; Pale, P.; Brenner, E.; Blanc, A. Chem. Eur. J. 2025, e202404446.
Pre-print: 10.26434/chemrxiv-2024-qrnt9
2024
Di, X.; Tony Garnier, T.; Clerc, A.; Jung, E.; Lherbet, C.; Bénéteau, V.; Pale, P.; Chassaing, S. Molecules 2024, 29, 5552.
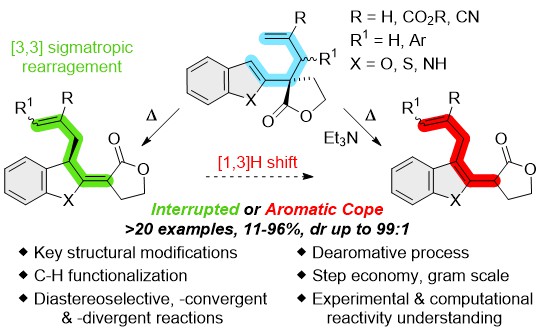
Unlocking the Aromatic Cope Rearrangement with Gold(I) Catalysis
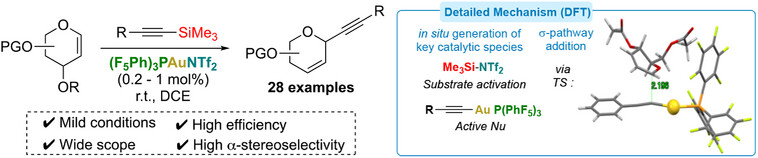
Locquet, P.; Rajamani, A.; Pereira, P.; Grellepois, F.; Weibel, J.-M.; Hénon, E.; Riguet, E.; Blanc, A. ACS Catal. 2024, 14, 18884–18895.
Pre-Print: 10.26434/chemrxiv-2024-6864j
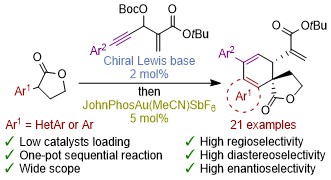
Strack, E.; Pascaretti, M.; Taillier, C.; Blanc, A.; Dalla, V.; Pale, P.; Weibel, J.-M. ACS Catal. 2024, 14, 14863–14870.
Lang, M.; Walter, S.; Hatey, D.; Blanc, A.; Compain, P.; Kern, N. Org. Lett. 2024, 26, 8017–8022.
Revillo Imbernon, J.; Weibel, J.-M.; Ennifar, E.; Prévost, G.; Kellenberger, E. Mol. Inform. 2024, e202300339.
Mando, M.; Grellepois, F.; Blanc, A.; Hénon, E.; Riguet, E. Chem. Eur. J. 2024, e202304138.
2023
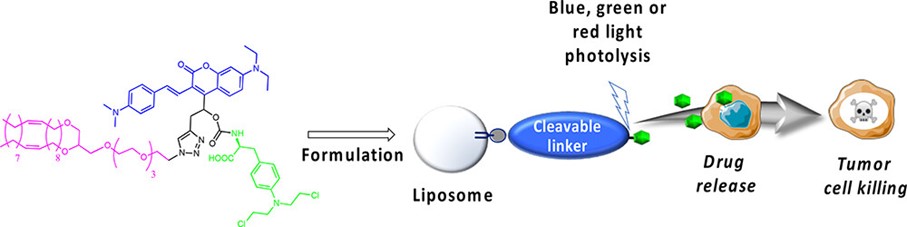
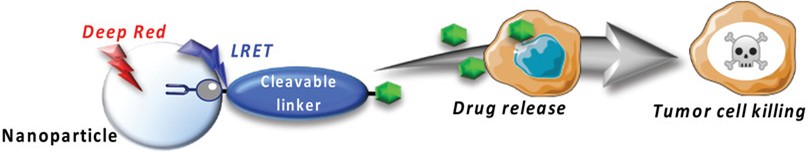
Brion, A.; Chaud, J.; Klimezak, M.; Ohlmann, L., Léonard, J.; Chassaing, S.; Frisch, B.; Kichler, A.; Heurtault, B.; Specht, A. Bioconjugate Chem., 2023, 34, 1304.
Brion, A.; Chaud, J.; Léonard, J.; Bolze, F.; Chassaing, S.; Frisch, B.; Heurtault, B.; Kichler, A.; Specht, A. Adv. Healthcare Mater., 2023, 12, 2201474.
Kvande, K.; Prodinger, S.; Schlimpen, F.; Beato, P.; Pale, P.; Chassaing, S.; Svelle, S. Topics in Catalysis, 2023, 66, 1406.
2022
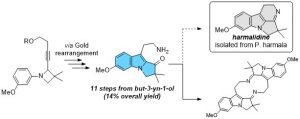
A Gold(I)-Catalysed Approach towards Harmalidine, an Elusive Alkaloid from Peganum Harmala
Miaskiewicz, S.; Weibel, J.-M.; Pale, P.; Blanc, A. RSC Advances, 2022, 12, 26966.
Dinuclear Silver(I) and Gold(I) Complexes with Chiral Oxazoline-NHC Ligands
Hoffmann, M.; Dargorne, S.; Pale, P.; Blanc, A.; de Frémont, P. J. Organomet. Chem., 2022, 979, 122507.
, T.; Bénéteau, V.; Pale, P.; Chassaing, S. Green Chem. 2022, 6467.
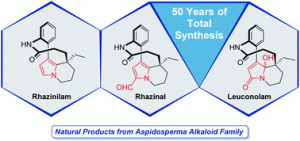
Rhazinilam-Leuconolam Family of Natural Products: a Half Century of Total Synthesis
Sirindil, F.; Weibel, J.-M.; Pale, P.; Blanc, A. Nat. Prod. Rep. 2022, 39, 1574.
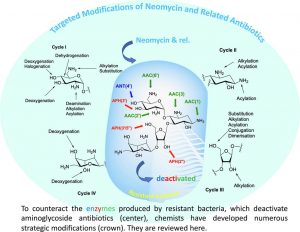
Targeted Modifications of Neomycin and Paromomycin: Towards Resistance–free Antibiotics?
Obszynski, J.; Loidon, H.; Blanc, A.; Weibel, J.-M.; Pale, P. Bioorg. Chem., 2022, 126, 105824.
Sirindil, F.; Pertschi, R.; Naulin, E.; Hatey, D.; Weibel, J.-M.; Pale, P.; Blanc, A.; ACS Omega 2022, 1186.
2021
; Bénéteau, V.; Pale, P.; Chassaing, S. J. Org. Chem. 2021, 86, 16593.
Chaud, J.; Morville, C.; Bolze, F.; Garnier, D.; Chassaing, S.; Blond, G.; Specht, A. Org. Lett. 2021, 23, 7580.
Pertschi, R.; de Aguirre, A.; Pale, P.; Blanc, A.; Poblador Bahamonde, A. I. Helv. Chim. acta. 2021, 104, e2100133.
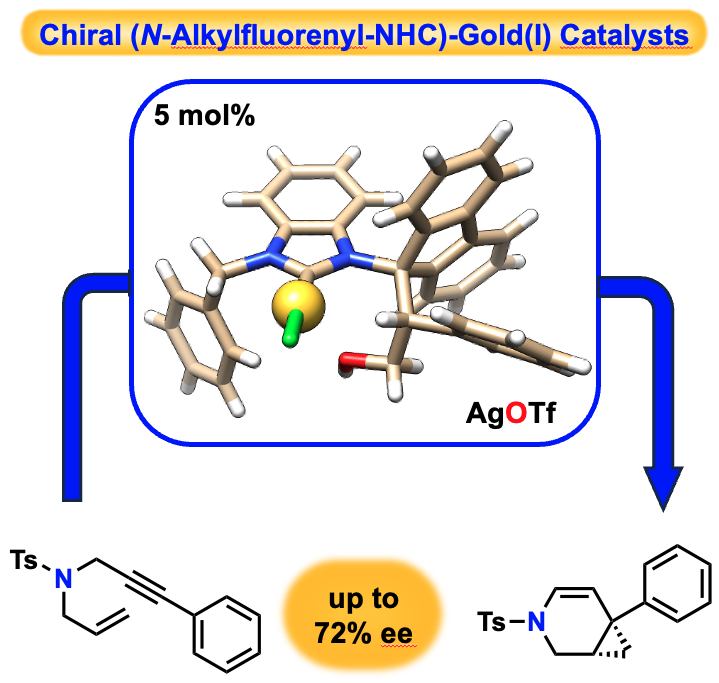
Pertschi, R.; Miaskiewicz, S.; Kern, N.; Weibel, J.-M.; Pale, P.; Blanc, A. Chem. Catal. 2021, 1, 129.
Recent Advances in the Synthesis and Applications of Chiral Gold(III) Complexes
Jouhannet, R.; Dagorne, S.; Blanc, A.; de Frémont, P. Chem.–Eur. J. 2021, 27, 9218.
Farjallah, A.; Chiarelli, L. R.; Forbak, M.; Degiacomi, G.; Danel, M.; Goncalves, F.; Carayon, C.; Seguin, C.; Fumagalli, M.; Záhorszká, M.; Vega, E. Abid, S.; Grzegorzewicz, A.; Jackson, M.; Antonio Peixoto, A.; Korduláková, J.; Pasca, M. R.; Lherbet, C.; Chassaing, S. ACS Infect. Dis. 2021, 7, 552.
2020
Peluso, P.; Sechi, B.; Lai, G.; Dessì, A.; Dallocchio, R.; Cossu, S.; Aubert, E.; Weiss, R.; Patrick Pale, P.; Mamane, V.; Bezhan Chankvetadze, B. J. Chromatogr. A 2020, 1625, 461303.
Peluso, P.; Mamane, V.; Dallocchio, R.; Dessì, A.; Cossu, S. J. Chromatogr. A 2020, 1623, 461202.
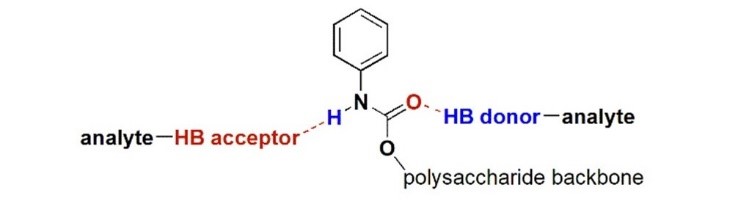
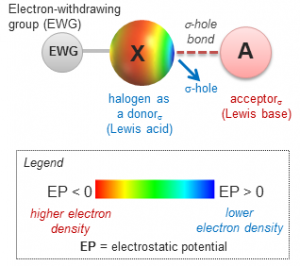
Halogen bond in separation science: overview, concepts and perspectives
Peluso, P.; Mamane, V.; Dessì, A.; Dallocchio, R.; Aubert, E.; Gatti, C.; Mangelings, D.; Sergio Cossu, S. J. Chromatogr. A 2020, 1616, 460788.
Dessì, A.; Peluso, P.; Dallocchio, R.; Weiss, R.; Andreotti, G.; Allocca, M.; Aubert, E.; Pale, P.; Mamane, V.; Cossu, S. Molecules 2020, 25, 2213.
Pertschi, R.; Hatey, D.; Pale, P.; de Frémont, P.; Blanc, A. Organometallic 2020, 39, 804–812.
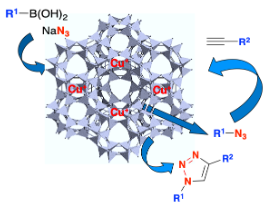
Evans‐Chan‐Lam‐Type Azidation and One‐Pot CuAAC under CuI‐Zeolite Catalysis
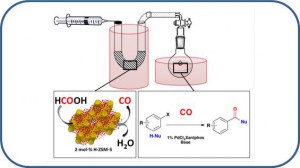
Clerc, A; Bénéteau, V; Pale, P; Chassaing, S. Chem. Cat. Chem. 2020, 12, 2060–2065.
2019
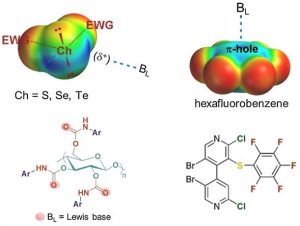
Chiral Chalcogen Bond Donors Based on the 4,4′-Bipyridine Scaffold
Weiss, R.; Aubert, E.; Peluso, P.; Cossu, S.; Pale, P.; Mamane, V. Molecules 2019, 24, 4484.
Synthesis of Indolizine and Pyrrolo[1,2-a]azepine Derivatives via a Gold(I)–Catalyzed 3-Step Cascade
Sirindil, F.; Golling, S.; Lamare, R.; Weibel, J. M.; Pale, P.; Blanc, A. Org. Lett. 2019, 21, 8997–9000.
Benzosultam Synthesis by Gold(I)-Catalyzed Ammonium Formation/Nucleophilic Substitution
Pertschi, R.; Weibel, J. M.; Pale, P.; Blanc, A. Org. Lett. 2019, 21, 5616–5620.
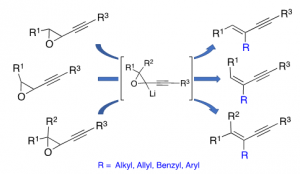
Borylation and rearrangement of alkynyloxiranes : a stereospecific route to substituted α-enynes.
Fuentespina R. P.; Garcia de la Cruz, J. L.; Durin, G.; Mamane, V.; Weibel, J.-M.; Pale, P. Beilstein J. Org. Chem. 2019, 15, 1416–1424.
Sirindil, F.; Weibel, J. M.; Pale, P.; Blanc, A. Org. Lett. 2019, 21, 5542–5546.
When Gold Cations Meet Polyoxometalates (Review)
Blanc, A.; de Frémont, P. Chem.–Eur. J. 2019, 25, 9553–9567.
2018
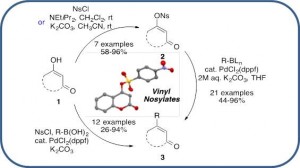
Vinyl Nosylates as Partner in Copper and Silver Co-Catalyzed Sonogashira Cross-Coupling Reactions
Cheval, N. P.; Hoffmann, B.; Dikova, A.; Sirindil, F., Blanc, A.; Weibel, J.-M.; Pale, P. Tetrahedron 2018, 74, 7111–7119.
Peluso, P.; Gatti, C.; Dessi, A.; Dallocchio, R.; Weiss, R.; Aubert, E.; Pale, P.; Cossu, S.; Mamane, V. J. Chromatogr. A, 2018, 119–129.
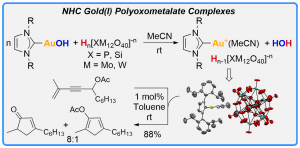
Synthesis, Characterization and Catalytic Activity of NHC Gold(I) Polyoxometalate Complexes
Sirindil, F.; Nolan, S. P.; Dagorne, S.; Pale, P.; Blanc, A.; de Frémont, P. Chem.–Eur. J. 2018, 24, 12630–12637.
Hueber, D.; Teci, M.; Brenner, E.; Matt, D.; Weibel, J.-M.; Pale, P.; Blanc, A. Adv. Synth. Catal. 2018, 360, 2453–2459.
Garnier, T.; Danel, M.; Magne, V.; Pujol, A.; Beneteau, V.; Pale, P.; Chassaing, S. J. Org. Chem. 2018, 83, 6408–6422.
Green Catalysts Based on Zeolites for Heterocycle Synthesis (Review)
Chassaing, S.; Beneteau, V.; Pale, P. Curr. Opin. Green Sustain. Chem. 2018, 10, 35–39.
Silver–Promoted Coupling Reactions (Book Chapter)
Weibel, J.-M.; Blanc, A.; Pale, P. Chapter 3.5.13 in Synthesis Knowledge Updates Vol. 3 Compounds of Groups 12 and 11, 2018/1, pp 1–109.
2017
Wimmer, E.; Borghèse, S.; Blanc, A.; Bénéteau, V.; Pale, P. Chem.–Eur. J. 2017, 23, 1484–1489.
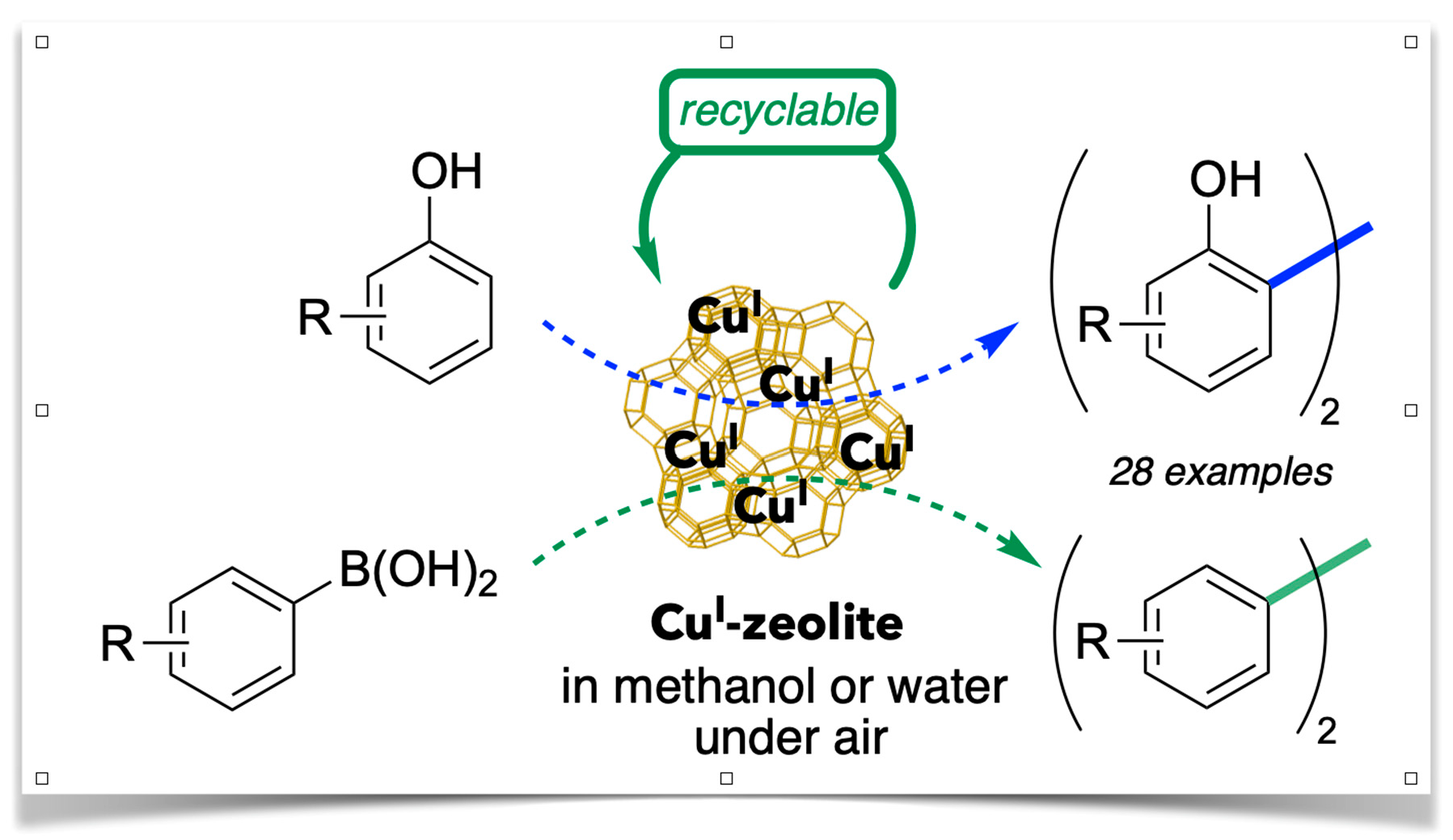
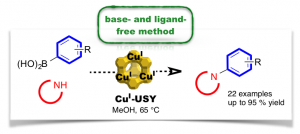
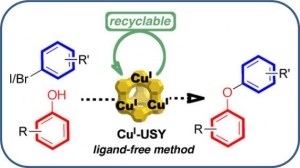
Chan–Lam-Type C–N Cross-Coupling Reactions under Base- and Ligand-Free CuI-Zeolite Catalysis
2016
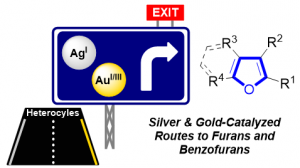
Silver & Gold Routes to Furans and Benzofurans (Review)
Blanc, A.; Bénéteau, V.; Weibel, J.-M.; Pale, P. Org. Biomol. Chem. 2016, 14, 9184–9205.
2015
Handy Protocols using Vinyl Nosylates in Suzuki–Miyaura Cross-Coupling Reactions
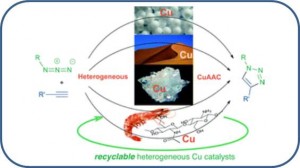
CuI–USY as a Ligand-Free and Recyclable Catalytic System for the Ullmann-Type Diaryl Ether Synthesis
Magné, V.; Garnier, T.; Danel, M.; Pale, P.; Chassaing, S. Org. Lett., 2015 17, 4494–4497.
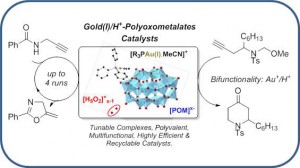
Polyoxometalate-Gold(I)/H+ Complexes: Air-Stable, Efficient, Polyvalent, and Bifunctional Catalysts
Hueber, D.; Hoffmann, M.; de Frémont, P.; Pale, P.; Blanc, A. Organometallics 2015, 34, 5065–5072.
Losch, P.; Felten, A.-S.; Pale, P. Adv. Synth. Catal. 2015, 357, 2931–2938.
Hoffmann, M.; Miaskiewicz, S.; Weibel, J.-M.; Pale, P.; Blanc, A. RCS Advances 2015, 5, 37138–37148.
2014
Zeo-Click Synthesis: Copper-Zeolite-Catalyzed Synthesis of Ynamides
Harkat, H.; Borghèse, S.; Nigris, M. D.; Kiselev, S.; Bénéteau, V.; Pale, P. Adv. Synth. Catal. 2014, 356, 3842–3848.
Kern, N.; Felten, A.-S.; Weibel, J.-M.; Pale, P.; Blanc, A. Org. Lett. 2014, 16, 6104–6107.
Copper(II) bromide as an efficient catalyst for acetal to bisarylmethyl ether interconversion
Mezaache, R.; Harkat, H.; Obszynski, J.; Benkouider, A.; Blanc, A.; Weibel, J.-M.; Pale, P. Tetrahedron Lett. 2014, 55, 7167–7171.
Specklin, S.; Dikova, A.; Blanc, A.; Weibel, J.-M.; Pale, P. Tetrahedron Lett. 2014, 55, 6987–6991.
Kern, N.; Hoffmann, M.; Weibel, J.-M.; Pale, P.; Blanc, A. Tetrahedron 2014, 70, 5519–5531.
Jacques, B.; Hueber, D.; Hameury, S.; Braunstein, P.; Pale, P.; Blanc, A.; de Frémont, P. Organometallics 2014, 33, 2326–2335.
Inorganic–Organic Heteropolyacid–Gold(I) Hybrids: Structures and Catalytic Applications
Hueber, D.; Hoffmann, M.; Louis, B.; Pale, P.; Blanc, A. Chem. – Eur. J. 2014, 20, 3903–3907.
Hoffmann, M.; Weibel, J.-M.; de Frémont, P.; Pale, P.; Blanc, A. Org. Lett. 2014, 16, 908–911.
2013
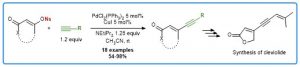
Gold(I)-catalyzed formation of furans from γ-acyloxyalkynyl ketones
Hoffmann, M.; Miaskiewicz, S.; Weibel, J.-M.; Pale, P.; Blanc, A. Beilstein J. Org. Chem. 2013, 9, 1774–1780.
Bernardon, C.; Louis, B.; Bénéteau, V.; Pale, P. ChemplusChem 2013, 78, 1134–1141.
Synthesis, Characterization, and Catalytic Activity of Cationic NHC Gold(III) Pyridine Complexes
Orbisaglia, S.; Jacques, B.; Braunstein, P.; Hueber, D.; Pale, P.; Blanc, A.; de Frémont, P. Organometallics 2013, 32, 4153–4164.
Vinyl Nosylates: An Ideal Partner for Palladium-Catalyzed Cross-Coupling Reactions
Cheval, N. P.; Dikova, A.; Blanc, A.; Weibel, J.-M.; Pale, P. Chem. – Eur. J. 2013, 19, 8765–8768.
Electrophilic chlorination of arenes with trichloroisocyanuric acid over acid zeolites
Mendonça, G. F.; Bastos, A. R.; Boltz, M.; Louis, B.; Pale, P.; Esteves, P. M.; de Mattos, M. C. S. Appl. Catal. Gen. 2013, 460-461, 46–51.
Genelot, M.; Cheval, N. P.; Vitorino, M.; Berrier, E.; Weibel, J.-M.; Pale, P.; Mortreux, A.; Gauvin, R. M. Chem. Sci. 2013, 4, 2680–2685.
Silver-zeolite catalysed solvent free synthesis of (spiro)ketals
Borghèse, S.; Drouhin, P.; Bénéteau, V.; Louis, B.; Pale, P. Green Chem. 2013, 15, 1496–1500.
Gold(I)-Catalyzed Rearrangement of N-Aryl 2-Alkynylazetidines to Pyrrolo[1,2-a]indoles
Kern, N.; Hoffmann, M.; Blanc, A.; Weibel, J.-M.; Pale, P. Org. Lett. 2013, 15, 836–839.
2012
Silver(I)-Catalyzed Deprotection of p-Methoxybenzyl Ethers: A Mild and Chemoselective Method
Kern, N.; Dombray, T.; Blanc, A.; Weibel, J.-M.; Pale, P. J. Org. Chem. 2012, 77, 9227–9235.
Green route for the chlorination of nitrobenzene
Boltz, M.; de Mattos, M. C. S.; Esteves, P. M.; Pale, P.; Louis, B. Appl. Catal. Gen. 2012, 449, 1–8.
Chassaing, S.; Specklin, S.; Weibel, J.-M.; Pale, P. Tetrahedron 2012, 68, 7245–7273.
Kern, N.; Blanc, A.; Miaskiewicz, S.; Robinette, M.; Weibel, J.-M.; Pale, P. J. Org. Chem. 2012, 77, 4323–4341.
Scandium(III)-Zeolites as New Heterogeneous Catalysts for Imino-Diels–Alder Reactions
Olmos, A.; Louis, B.; Pale, P. Chem. – Eur. J. 2012, 18, 4894–4901.
Vinyl and Aryl Sulfonates: Preparations and Applications in Total Synthesis (Review)
Chassaing, S.; Specklin, S.; Weibel, J.-M.; Pale, P. Curr. Org. Synth. 2012, 9, 806–827.
2011
Borghèse, S.; Blanc, A.; Pale, P.; Louis, B. Dalton Trans. 2011, 40, 1220–1223.
Specklin, S.; Gallier, F.; Mezaache, R.; Harkat, H.; Dembelé, Y. A.; Weibel, J.-M.; Blanc, A.; Pale, P. Tetrahedron Lett. 2011, 52, 5820–5823.
Design of silver(I)-heteropolyacids: toward the molecular control of reactivity in organic chemistry
Borghèse, S.; Louis, B.; Blanc, A.; Pale, P. Catal. Sci. Technol. 2011, 1, 981–986.
Rational Design of Microporous and Mesoporous Solids for Catalysis: From the Molecule to the Reactor
Louis, B.; Laugel, G.; Pale, P.; Pereira, M. M. ChemCatChem 2011, 3, 1263–1272.
Gold(I)-catalyzed rearrangement of aryl alkynylaziridines to spiro[isochroman-4,2′-pyrrolines]
Kern, N.; Blanc, A.; Weibel, J.-M.; Pale, P. Chem. Commun. 2011, 47, 6665–6667.
Olmos, A.; Sommer, J.; Pale, P. Chem. – Eur. J. 2011, 17, 1907–1914.
Amino-benzosuberone: A novel warhead for selective inhibition of human aminopeptidase-N/CD13
Albrecht, S.; Al-Lakkis-Wehbe, M.; Orsini, A.; Defoin, A.; Pale, P.; Salomon, E.; Tarnus, C.; Weibel, J.-M. Bioorg. Med. Chem. 2011, 19, 1434–1449.
2010
Gold(I)-Catalyzed Cycloisomerization of β-Alkynylpropiolactones to Substituted α-Pyrones
Dombray, T.; Blanc, A.; Weibel, J.-M.; Pale, P. Org. Lett. 2010, 12, 5362–5365.
Boningari, T.; Olmos, A.; Reddy, B. M.; Sommer, J.; Pale, P. Eur. J. Org. Chem. 2010, 2010, 6338–6347.
Copper(I)-Zeolites as New Heterogeneous and Green Catalysts for Organic Synthesis
Kuhn, P.; Louis, B.; Sommer, J.; Pale, P. Synthesis 2010, 1557–1567.
Bénéteau, V.; Olmos, A.; Boningari, T.; Sommer, J.; Pale, P. Tetrahedron Lett. 2010, 51, 3673–3677.
Metal-doped Zeolites as Green Catalysts for Organic Synthesis
Chassaing, S.; Alix, A.; Olmos, A.; Keller, M.; Sommer, J.; Pale, P. Z. Naturforsch. B 2010, 65, 783–790.
Blanc, A.; Alix, A.; Weibel, J.-M.; Pale, P. Eur. J. Org. Chem. 2010, 2010, 1644–1647.
Besbes, N.; Jellali, H.; Pale, P.; Srasra, E.; Efrit, M. L. Comptes Rendus Chim. 2010, 13, 358–364.