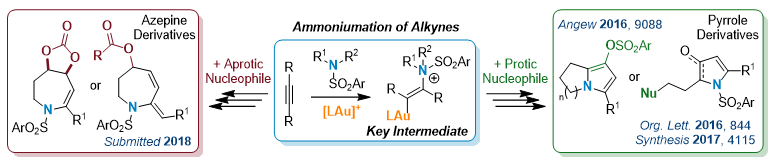
Cascade Reactions via Gold-Catalyzed Ammoniumation1
With their exceptional carbophilic acidity, cationic gold(I) complexes are the catalysts of choice in the activation of unsaturated carbon-carbon bonds for nucleophilic addition. In this area, we have recently disclosed the efficiency of gold to promote the addition of secondary sulfonamides, as primary nucleophile, onto alkynes toward the preparation of elaborated nitrogenous compounds. The outcome of the reaction is efficiently controlled by the nature of a second nucleophile used to interact with the key vinyl-gold ammonium intermediate.
Multifaceted-Gold Catalysis
Gold cations are not only carbophilic (π) Lewis acid, they also exhibit a significant oxophilic character (σ Lewis).2 This double behavior offers the possibility to develop new synthetic methods via a “multifaceted catalysis” process. Therefore, taking benefit of the both s and p Lewis acidities, various valuable compounds were obtained starting from alkynyl-oxirane, -aziridine or -azetidine derivatives in the presence of internal or external nucleophiles.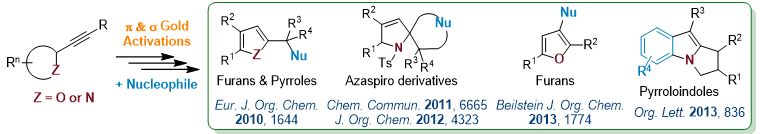
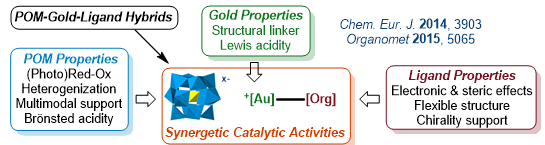
Polyoxometalate/Gold/Ligand Hybrids as Multifunctional Catalysts
With Green Chemistry principles in mind (notably recyclability, atom economy, limiting waste and catalytic processes), this topic is aiming at efficiently prepare innovative materials issued from the assemblies of polyoxometalates (POM)/gold(I/III)/organic ligands and apply them as new synergetic catalysts in relevant organic transformations.
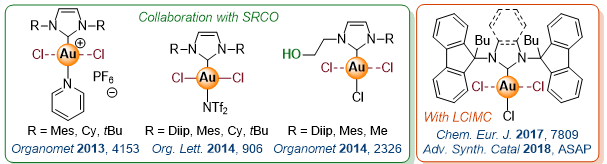
Development of N-Heterocyclic Carbenes (NHC) Gold(I/III) Catalysts
In the collaboration with Dr Pierre de Fremont, we are designing new NHC chiral or achiral gold complexes for specific catalytic applications.